Abstract
COVID-19 vaccination during pregnancy is safe and effective in reducing the risk of complications. However, the uptake is still below targets worldwide. This study aimed to explore the factors associated with COVID-19 vaccination uptake among pregnant women since data on this topic is scarce in low-to-middle-income countries. A retrospective cohort study included linked data on COVID-19 vaccination and pregnant women who delivered a singleton live birth from August 1, 2021, to July 31, 2022, in Rio de Janeiro City, Brazil. Multiple logistic regression was performed to identify factors associated with vaccination during pregnancy, applying a hierarchical model and describing odds ratio with 95% confidence intervals. Of 65,304 pregnant women included in the study, 53.0% (95% CI, 52–53%) received at least one dose of COVID-19 vaccine during pregnancy. Higher uptake was observed among women aged older than 34 (aOR 1.21, 95%CI 1.15–1.28), black (aOR 1.10, 1.04–1.16), or parda/brown skin colour (aOR 1.05, 1.01–1.09), with less than eight years of education (aOR 1.09, 1.02–1.17), living without a partner (aOR 2.24, 2.16–2.34), more than six antenatal care appointments (aOR 1.92, 1.75–2.09), and having a previous child loss (OR 1.06, 1.02–1.11). These results highlight the need for targeted educational campaigns, trustful communication, and accessibility strategies for specific populations to improve vaccination uptake during pregnancy.
Introduction
COVID-19 during pregnancy and puerperium is associated with higher morbidity and mortality risk, including hospitalisation, use of respiratory support, and admission to an intensive care unit when compared to non-pregnant pairs1,2,3. In 2021 in Brazil, for example, the estimated COVID-19 case fatality rate among pregnant and puerperal women was 7.2%, compared to 2.8% in the general population4. Furthermore, COVID-19 has been linked to adverse perinatal events, such as preterm birth, fetal loss, and neonatal mortality2, 5.
COVID-19 vaccination in pregnant women is a safe and effective preventive measure that can reduce the risk of COVID–19–associated complications for both mother and child6,7,8,9,10,11. However, COVID-19 uptake is still below targets worldwide. According to a systematic review, in 2021, the average uptake by the time of delivery was 27.5%12, reaching 78% in Sweden and 87% in Norway13. In addition, most studies evaluating COVID-19 vaccination among pregnant women identified older age, white self-identified skin colour, high level of education, gravidity, and high socioeconomic status as factors associated with vaccination, predominantly in high-income countries9, 12,13,14.
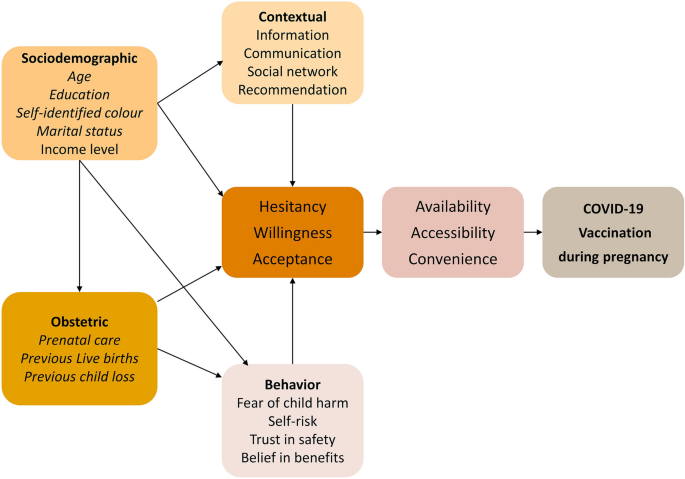
Based on the literature, we developed a conceptual framework describing the potential factors associated with COVID-19 vaccination during pregnancy (Fig. 1)9, 12, 15,16,17,18,19. Sociodemographic aspects, obstetric history, social context, beliefs, and behaviours can contribute to the mother’s acceptance and vaccination uptake and therefore were included in the conceptual framework.
Identifying factors associated with COVID-19 vaccine uptake in pregnant women is relevant to support public health decision-making and improve vaccine equity and coverage while addressing regional health disparities related to age, skin colour, education level, income, and access to antenatal care. However, these data on associated factors, especially from low-middle-income countries, are still scarce. Therefore, this study investigates the sociodemographic and obstetric factors that can contribute to COVID-19 vaccine uptake during pregnancy in Brazil.
Results
Study population
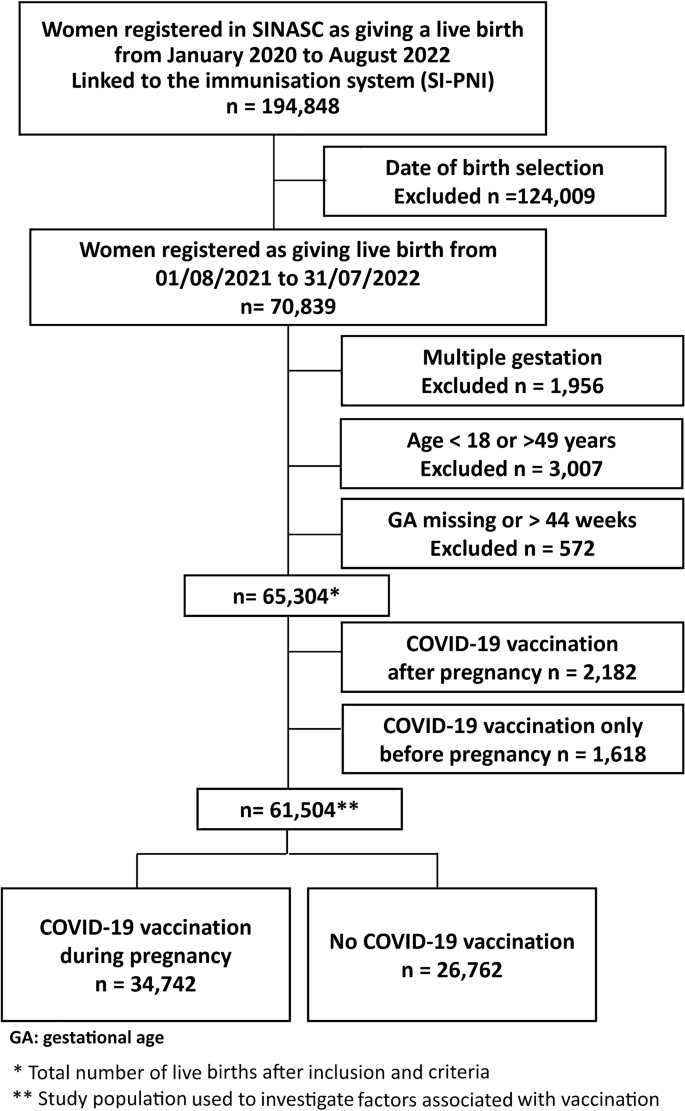
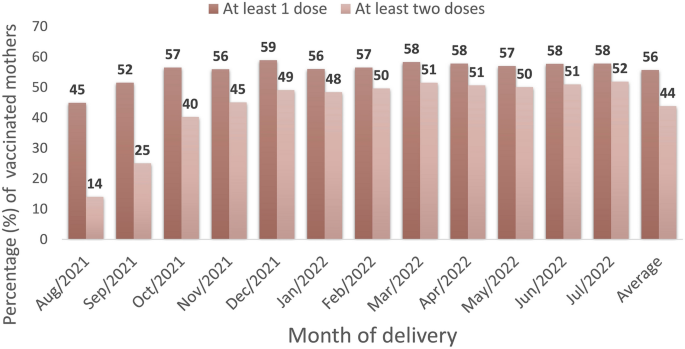
During the study period, 65,304 singleton live births from women aged 18–49 years, with gestational age between 22 and 44 weeks, were registered in Rio de Janeiro city. The schematic diagram describes our data selection process and the study population (Fig. 2; Supplementary Figure 1 and Table 3). About 56% (95% CI, 55–56%) of our study population received at least one dose of the COVID-19 vaccine, and 44% (95% CI, 43–44%) at least two doses up to delivery, including those vaccinated before and during pregnancy (Fig. 3; Supplementary Table 4). The COVID-19 vaccine uptake during pregnancy, with at least one dose, was 53% (34,742/65,304; 95% CI, 52-53%) (Supplementary Table 3). Of those mothers vaccinated during pregnancy, the platforms most used were BNT162b2 (Pfizer/Biotech) (68%) and CoronaVac (Sinovac/Butantan) (27%) in at least one dose, mainly during the second and third trimesters of pregnancy (Supplementary Tables 5 and 6).
Table 1 describes the characteristics of those vaccinated before pregnancy, during pregnancy, and those not vaccinated during the study period. Women vaccinated only before pregnancy differed slightly from those vaccinated during pregnancy (Table 1). They had higher proportions of age > 34 years (34% × 22%), at least 12 years of education (45% × 28%), self-identified white skin colour (40% × 32%), living with a partner (42% × 28%), and a higher proportion of a previous child loss (26% × 23%). Most of the women in the three groups started antenatal care in the first trimester and had more than six visits. Women who were never vaccinated, when compared with those vaccinated during pregnancy, were more likely to have 12 years or more of education (37%× 28%), self-identified as white (38% × 32%), and live with a partner (47% × 29%) (Table 1).
Factors associated with vaccine uptake
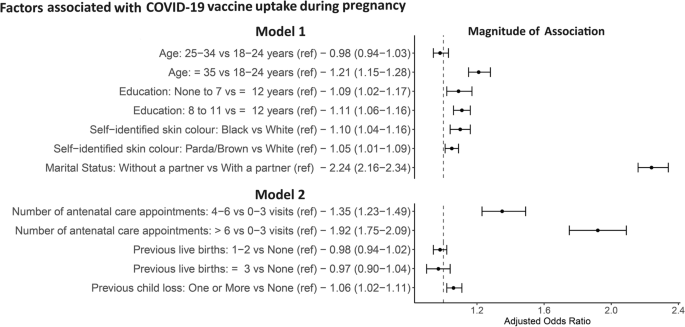
In an unadjusted analysis, age, education level, self-identified skin colour, antenatal care, number of live births, and previous child loss were potentially associated with COVID-19 vaccination during pregnancy (Supplementary Table 7). In the first model of the multiple logistic regression (including the block of socioeconomic variables and confounders factors), women older than 34 years (aOR 1.21, 95% CI 1.15–1.28) were more likely to be vaccinated than those younger than 25. In addition, women living without a partner (aOR 2.24, 95% CI 2.16–2.34), of black (aOR 1.10, 95% CI 1.04–1.16) or parda/brown (aOR 1.05, 95% CI, 1.01–1.09) self-identified skin colour, with a low level of education (0-7 years aOR 1.09, 95% CI 1.02–1.17; 8–11 years aOR 1.11, 95% CI, 1.06–1.16) were more likely to be vaccinated than white women, with 12 years or more of education, living with a partner (Fig. 4 and Supplementary Table 7).
Forest plot of the magnitude of association between the factors associated with the uptake of the COVID-19 vaccine during pregnancy. Ref: variable used as the reference in the multiple logistic regression. Model 1: included socioeconomic (age, education, self-identified skin colour, marital status) Model 2: included obstetric (the total number of antenatal care appointments, the number of previous live births, the number of previous child loss) and socioeconomic.Month-year of birth and the gestational week at delivery are included as confounding variables in both models.
In the second model (including obstetric variables in the presence of socioeconomic variables and confounders), the likelihood of vaccination increased progressively with the number of antenatal care appointments compared to less than 4 (4-6 aOR 1.35, 95% CI 1.23-1.49; > 6 aOR 1.92, 95% CI 1.75-2.09). Having at least a previous child loss (aOR 1.06, CI 95% 1.02-1.11) was a factor potentially associated with vaccination during pregnancy, compared to none. The number of previous live births was not associated with uptake among pregnant women (Fig. 4 and Supplementary Table 7).
Discussion
This study estimated COVID-19 vaccine uptake during pregnancy as 53%, with most pregnant women receiving Pfizer and CoronaVac vaccines. The likelihood of a COVID-19 vaccine uptake was higher among women of older age, living without a partner, and with more antenatal care appointments. With lower magnitude, self-identify as black and brown skin colour, low educational level, and a previous child loss were also associated with vaccine uptake in this population.
Despite the well-defined maternal and neonatal COVID-19 risks and the vaccine’s proven benefits, the vaccine uptake among pregnant women was lower than among the general population, which was 89%. Yet, it was similar to or higher than the COVID-19 vaccine uptake during pregnancy in different countries, for example, the United Kingdom (32–66%), the United States (16–22%), Palestine (25%), and Mexico (20%)8, 14, 20,21,22,23,24.
In the cohort study presented herein, women older than 34 had a 21% higher chance of being vaccinated than those younger than 25, consistent with previous studies12, 25, 26. We also found higher odds of vaccination among pregnant women with less than 12 years of education when compared to less than eight, contrary to the findings of other studies conducted in middle-high-income countries13, 15, 16, 20, 24, 27 but similar to a Chinese survey that observed low acceptance of COVID-19 vaccination in women with a high level of education25. The authors hypothesise that this group could be more concerned about safety and side effects25.
In our population, black and parda/brown skin colour were associated with COVID-19 vaccine uptake during pregnancy; however, the majority of studies pointed to black, non-white and Hispanic races as less likely to be vaccinated than white 13, 15, 16, 20, 24, 26, 27. These conflicting findings on self-identified skin colour and education could be related to Brazilian social inequities, regional diversity, the political context, and the accessibility to vaccines only available in the public health system. This enables the access of pregnant women to vaccination while they attend public antenatal care, probably receiving the recommendation and the shot at the same visit. Historically, the Unified Public Health system assists proportionally more people from lower income levels, from black and brown self-identified colour, and with low educational levels 28; this could explain higher uptake in these groups.
Pregnant women without a partner were more than twice as likely to be vaccinated than those living with a partner in our study. This contrasts with findings among women of reproductive age in the US, for which being single was associated with 29% lower COVID-19 vaccination likelihood 29. Moreover, single mothers from low-middle-income countries are more frequently the economic providers of the family. Thus, their own decision to vaccinate to prevent getting ill could be considered a way of avoiding absenteeism and maintaining their work capacity and employment during the COVID-19 pandemic21, 30.
Previous studies showed that more antenatal care appointments, prenatal nurse follow-ups, and being counselled about vaccination by a health care provider increase the likelihood of vaccination during pregnancy. Conversely, antenatal care onset after the first quarter of pregnancy decreases the uptake31,32,33. These findings demonstrate the impact of early and complete antenatal care as an essential measure for vaccine education, recommendation, and accessibility during pregnancy. Rio de Janeiro’s well-established maternal health program maintains more than 80% of women on early and complete antenatal care. Additionally, a history of miscarriage may influence the perception of COVID-19 susceptibility, severity, and vaccine benefits21, 32. Therefore, women with a history of fetal loss could have been more preventive during their pregnancy, hence, accepting the vaccination as a protection for themselves and the unborn child.
To note, we also described the subset of women vaccinated only before pregnancy as having a higher proportion of older age, white self-identified skin colour, and more than 12 years of education; however, this group wasn’t the objective of this study. It is possible that these women were prioritised to receive the COVID-19 vaccine earlier due to pre-existing conditions, high risk of complications, or increased exposure as healthcare workers.
The high quality of the Brazilian data, the population size and the breadth of data are strengths of this study. Furthermore, it is one of the first studies to address factors associated with COVID-19 vaccine uptake in pregnant women in Brazil and provide new evidence of low vaccine uptake in this population.
This study has some limitations, particularly related to the use of secondary data sources. Routinely administrative data are not collected for the research proposal and have limited information available. Therefore, we were not able to study other important variables, such use of private insurance. Linkage error is another potential limitation. Although linkage errors can occur, and some women may be more likely to link than others, the linkage approach was bespoke, and a manual review was performed to improve linkage accuracy. Additionally, it is important to note that incomplete data can occur. However, the amount of missing data for each variable was relatively low (Supplementary Table 8).
Our study did not include pregnant women who had an abortion, a stillbirth, or multiple gestations; therefore, the results presented in this study cannot be generalized to these groups. Wide misinformation released on social media, concerns regarding vaccine safety, scepticism about the disease risk and the political context could have influenced the uptake among pregnant women. Yet, they were not addressed herein and should be better investigated in further studies15, 34,35,36. Moreover, factors associated with vaccine uptake may vary from one place to another; therefore, it cannot be generalised to other Brazilian municipalities.
In conclusion, COVID-19 vaccination uptake remains poor among pregnant women in Brazil. There is a higher uptake among those older than 34 years, of black or brown self-identified skin colour, with a low level of education, living without a partner, having more than six appointments, and with a previous child loss in Rio de Janeiro city. These findings contribute to planning targeted educational campaigns, trustful communication, and accessibility strategies to specific populations to improve vaccine uptake since pregnant women widely benefit from COVID-19 vaccination. Factors associated with vaccination likely vary from one place to another and by population; therefore, further local studies are needed to guide intervention and address the impact of socioeconomic, socio-political, and social media on immunisation uptake.
Methods
Study setting, study design, and population
This retrospective cohort study used linked data from the live birth and vaccination information systems in Rio de Janeiro city. Rio de Janeiro is the second largest city in Brazil, with an estimated population of 6,700,000. It has a monthly per capita income of 4 minimum wage, considered a high-income city compared to other regions of the country37 In 2021, the number of live births was 67,97338. In July 2022, the coverage of COVID-19 vaccination was 89% for the complete primary regimen and 57% for a booster dose in the general population above 18 years39.
The COVID-19 immunisation started in Rio de Janeiro city on January 20, 202140, initially for healthcare professionals, adults older than 60 years, and high-risk groups. Pregnant women with comorbidities started vaccination in March 2021; by May 2021, it was interrupted due to a severe adverse event related to the COVID-19 vaccine in this population. From July 07, 2021, vaccination was resumed and made available for all pregnant, breastfeeding or women planning to become pregnant41. CoronaVac (Sinovac/Butantan) and BNT162b2 (Pfizer/Biotech) were the recommended platforms.
For this investigation, we included all women who delivered live births in Rio de Janeiro city between 1st August 2021 and 31 July 2022. Our exclusion criteria were multiple births, ages less than 18 years or higher than 49 years, and records with missing or implausible gestational ages (> 44 weeks) (Fig. 2).
Data sources and linkage process
The Declaration of Live Birth, a legal document filled out by the health care professional who attends the birth, is entered into the Live Birth Information System (SINASC). It contains details on the mother (such as age, education, skin colour, and marital status), about the pregnancy (such as antenatal appointments, the gestational period, prior gestations, previous live births, and previous losses), and details about the newborn (e.g., birth weight, sex, APGAR score). In addition, all vaccinations provided in Brazil are documented in the National Immunization Program Information System (SI-PNI), along with the administration date of the first, second, and booster doses, with its platform type. The SINASC data initially available had records from women who gave a live birth in Rio de Janeiro city from January 1, 2020, to August 28, 2022. The SI-PNI data included vaccination records from January 19, 2021, to August 31, 2022 (Supplementary Figure 1, Table 1 and Table 2). The linkage between records from SI-PNI and SINASC allowed access to any vaccination that happened before, during and after the pregnancy period.
The matching process used the maternal name, date of birth, zip code, and neighbourhood. We used the Jaro-Winkler string comparator to compare the similarity between string variables recorded in SINASC and SI-PNI. This algorithm calculates the similarity between two strings based on the number of shared characters and transpositions42. The resulting similarity score ranges between 0 (no similarity) and 1 (perfect similarity). We categorised the similarity scores into three categories: (0, 0.85), (0.85, 0.95), and (0.95, 1)]. We employed a three-step approach that checked for a string similarity score greater than 0.95, followed by exact matches for dates of birth and zip code. Any potential matches then underwent a manual review. After data linkage, the individual identifiers were removed, and the de-identified dataset was made available for analysis.
COVID-19 vaccine status
We estimated the date of the last period (DLP) using the date of delivery minus the days of pregnancy according to the gestational age at birth. We determined the date of conception by adding 14 days to the DLP. We defined the gestational period as the time between the date of conception and the date of birth. The vaccination status was determined using the dates of the vaccination registries compared to the gestational period.
Women who received at least one dose between the conception date and the date of delivery were considered vaccinated during pregnancy. Those who received all registered doses before the pregnancy period (before the conception date) were grouped as vaccinated before pregnancy. Those who received vaccines exclusively after the delivery date were assigned as vaccinated after pregnancy. Finally, women with no register of a vaccine dose were regarded as never vaccinated. We estimated vaccine uptake during pregnancy as the proportion of women who received any vaccination during pregnancy as a percentage of all births (Supplementary Table 3).
Variables
We divided variables into sociodemographic: age (18–24, 25–34, ≥35 years), education (0–7, 8–11, ≥12 years), self-identified skin colour (black, parda/brown, white, Asian, or Indigenous), and marital status (with or without a partner). And obstetric: the trimester of the first antenatal care appointment (first, second, or third), the total number of appointments (0–3, 4–6, or >6), the number of previous gestations (none, 1–2, or ≥3), the number of previous live births (none, 1–2, or ≥3), and the number of previous child loss (none or at least one). The Indigenous and Asian races were presented in the descriptive analysis and excluded from the logistic regression due to the small sample size.
We considered the length of pregnancy and the burden of COVID-19 infection during the study period to be a confounder a priori. Therefore, we included the month-year of birth and the gestational week at delivery as additional variables in the analyses.
Statistical analysis
We assessed each group’s characteristics by describing categorical variables as frequencies and percentages, excluding missing data. Continuous variables, such as age and gestational age, were presented as the median and interquartile range (IQR). In the descriptive analysis, we stratified the groups by being vaccinated only before pregnancy, vaccinated with at least one dose during pregnancy, and never vaccinated.
To identify the factors associated with vaccine uptake in pregnant women, we compared only the population of women vaccinated during pregnancy with those who were never vaccinated during the study period. For each potential factor associated with uptake, we ran a bivariate logistic regression individually, describing the crude odds ratio (OR) and its associated 95% confidence interval, controlled by gestational age and month-year of birth (Supplementary Table 7).
In addition, we performed a multiple logistic regression using a hierarchical framework with two levels. In the first level, we had the socioeconomic variables: age, education, self-identified skin colour, and marital status. In the second level, we included all the variables above and the obstetrics variables: the total number of antenatal appointments, the number of previous live births, and the number of previous child losses.
In the first model of multiple logistic regression, we included only the socioeconomic variables. The overall effect of socioeconomic factors (the distal factors) was assessed in this model 1. In the second model, we included the obstetric variables in addition to the sociodemographic block. Therefore, the unconfounded effect of the obstetric variables was obtained in this model. Both models were also controlled by gestational age and month-year of birth. Missing data on each covariate were excluded from the analysis (Supplementary Table 8). The final adjusted odds ratio and 95% confidence intervals were described for each model separately (Supplementary Table 7). Data management and statistical analysis were conducted using IBM SPSS, Statistical Package for the Social Sciences, Version 28.0 (Armonk, NY: IBM Corp). The de-identified dataset and the programming codes can be made available under the request.
Ethics
The present study has been approved by the Ethics Committee of Research Center Gonçalo Moniz /Oswaldo Cruz Foundation, Salvador, Bahia, Brazil (IORG 0002090/OMB No. 0990-0279 valid until 01/27/2025), under the Certificate of Submission for Ethics Review No 63287822.0.0000.0040. The protocol and procedures presented in the project are in full accordance with the Brazilian legislation (Resolution CNS 466/2012) and the declaration of Helsinki regarding ethical standards in conducting research involving human beings. Due to the retrospective nature of the study, the need for informed consent was waived by the Ethics Committee of Research Center Gonçalo Moniz /FIOCRUZ/BA.
Data availability
All raw data from the information systems are available at: http://sistemas.saude.rj.gov.br/tabnet/tabcgi.exe?sinasc/nascido.def –http://sistemas.saude.rj.gov.br/tabnetbd/dhx.exe?pni_covid/pni_covid.def. The de-identified linkage dataset and the programming codes could be made available under the request by contacting the corresponding author MASBB.
References
-
Maza-Arnedo, F. et al. Maternal mortality linked to COVID-19 in Latin America: results from a multi-country collaborative database of 447 deaths. Lancet Reg. Health Am. 12, 100269. https://doi.org/10.1016/j.lana.2022.100269 (2022).
-
Smith, E. R. et al. Adverse maternal, fetal, and newborn outcomes among pregnant women with SARS-CoV-2 infection: an individual participant data meta-analysis. BMJ Glob. Health 8, e009495. https://doi.org/10.1136/bmjgh-2022-009495 (2023).
-
Safadi, M. A. P., Spinardi, J., Swerdlow, D. & Srivastava, A. COVID-19 disease and vaccination in pregnant and lactating women. Am. J. Reprod Immunol. 88, e13550. https://doi.org/10.1111/aji.13550 (2022).
-
Observatório Covid-19. Boletim extraordinário COE-Covid-19. (Fundação Oswaldo Cruz, Rio de Janeiro, Brazil). <https://agencia.fiocruz.br/sites/agencia.fiocruz.br/files/u34/boletim_extraordinario_2021-junho-23-parte2-pags09-17.pdf> (2021).
-
Sturrock, S., Ali, S., Gale, C., Battersby, C. & Le Doare, K. Neonatal outcomes and indirect consequences following maternal SARS-CoV-2 infection in pregnancy: a systematic review. BMJ Open 13, e063052. https://doi.org/10.1136/bmjopen-2022-063052 (2023).
-
Florentino, P. T. V. et al. Safety of BNT162b2 and CoronaVac during pregnancy on birth outcomes and neonatal mortality: a cohort study from Brazil. International Journal of Epidemiology, dyad120, doi: https://doi.org/10.1093/ije/dyad120 (2023).
-
Halasa, N. B. et al. Effectiveness of maternal vaccination with mRNA COVID-19 vaccine during pregnancy against COVID-19-associated hospitalization in infants Aged <6 Months - 17="17" States>MMWR. Morbidity and Mortality Weekly Rep. 71, 264–270. https://doi.org/10.15585/mmwr.mm7107e3 (2022).
-
Lipkind, H. S. et al. Receipt of COVID-19 vaccine during pregnancy and preterm or small-for-gestational-age at birth – eight integrated health care organizations, United States, December 15, 2020-July 22, 2021. MMWR. Morbidity and Mortality Weekly Rep. 71, 26–30. https://doi.org/10.15585/mmwr.mm7101e1 (2022).
-
Rawal, S., Tackett, R. L., Stone, R. H. & Young, H. N. COVID-19 vaccination among pregnant people in the United States: a systematic review. Am. J. Obstetrics & Gynecol. MFM 4, 100616. https://doi.org/10.1016/j.ajogmf.2022.100616 (2022).
-
Watanabe, A. et al. Peripartum outcomes associated with COVID-19 vaccination during pregnancy: a systematic review and meta-analysis. JAMA Pediatrics 176, 1098–1106. https://doi.org/10.1001/jamapediatrics.2022.3456 (2022).
-
Ellington, S. & Jatlaoui, T. C. COVID-19 vaccination is effective at preventing severe illness and complications during pregnancy. The Lancet 401, 412–413. https://doi.org/10.1016/S0140-6736(22)02613-7 (2023).
-
Galanis, P. et al. Uptake of COVID-19 vaccines among pregnant women: a systematic review and meta-analysis. Vaccines https://doi.org/10.3390/vaccines10050766 (2022).
-
Örtqvist, A. K. et al. COVID-19 vaccination in pregnant women in Sweden and Norway. Vaccine 40, 4686–4692. https://doi.org/10.1016/j.vaccine.2022.06.083 (2022).
-
Stock, S. J. et al. SARS-CoV-2 infection and COVID-19 vaccination rates in pregnant women in Scotland. Nat Med 28, 504–512. https://doi.org/10.1038/s41591-021-01666-2 (2022).
-
Blakeway, H. et al. COVID-19 vaccination during pregnancy: coverage and safety. Am. J. Obstet. Gynecol. 226(236), e231-236.e214. https://doi.org/10.1016/j.ajog.2021.08.007 (2022).
-
Kiefer, M. K. et al. Characteristics and perceptions associated with COVID-19 vaccination hesitancy among pregnant and postpartum individuals: a cross-sectional study. BJOG 129, 1342–1351. https://doi.org/10.1111/1471-0528.17110 (2022).
-
Nichol, B. et al. Barriers and facilitators of vaccine hesitancy for COVID-19, influenza, and pertussis during pregnancy and in mothers of infants under two years: an umbrella review. PLOS ONE 18, e0282525. https://doi.org/10.1371/journal.pone.0282525 (2023).
-
Razzaghi, H. et al. COVID-19 vaccination and intent among Pregnant Women, United States, April 2021. Public Health Rep. 137, 988–999. https://doi.org/10.1177/00333549221099244 (2022).
-
Azami, M., Nasirkandy, M. P., Esmaeili Gouvarchin Ghaleh, H. & Ranjbar, R. COVID-19 vaccine acceptance among pregnant women worldwide: A systematic review and meta-analysis. PLOS ONE 17, e0272273, doi:https://doi.org/10.1371/journal.pone.0272273 (2022).
-
Husain, F. et al. COVID-19 vaccination uptake in 441 socially and ethnically diverse pregnant women. PLOS ONE 17, e0271834. https://doi.org/10.1371/journal.pone.0271834 (2022).
-
Nazzal, Z. et al. Coverage and determinants of COVID-19 vaccination among pregnant women: an experience from a low-income country. Am. J. Health Promotion 37, 222–227. https://doi.org/10.1177/08901171221111107 (2022).
-
Rodriguez-Sibaja, M. J. et al. COVID-19 vaccination uptake among pregnant individuals in a middle-income setting. Int. J. Gynaecol. Obstet. 159, 607–609. https://doi.org/10.1002/ijgo.14344 (2022).
-
Mhereeg, M. et al. COVID-19 vaccination in pregnancy: views and vaccination uptake rates in pregnancy, a mixed methods analysis from SAIL and the Born-In-Wales Birth Cohort. BMC Infectious Dis. 22, 932. https://doi.org/10.1186/s12879-022-07856-8 (2022).
-
Razzaghi, H. et al. COVID-19 vaccination coverage among pregnant women during pregnancy – eight integrated health care organizations, United States, December 14, 2020-May 8, 2021. MMWR. Morbidity and Mortality Weekly Rep. 70, 895–899. https://doi.org/10.15585/mmwr.mm7024e2 (2021).
-
Tao, L. et al. Acceptance of a COVID-19 vaccine and associated factors among pregnant women in China: a multi-center cross-sectional study based on health belief model. Hum. Vaccin. Immunother. 17, 2378–2388. https://doi.org/10.1080/21645515.2021.1892432 (2021).
-
de Andrade Pereira Silva, M. et al. Factors associated with vaccination against Covid-19 in pregnant and hospitalized postpartum women: a retrospective cohort study. PLOS ONE 17, e0269091. https://doi.org/10.1371/journal.pone.0269091 (2022).
-
Egloff, C. et al. Pregnant women’s perceptions of the COVID-19 vaccine: a French survey. PLoS One 17, e0263512. https://doi.org/10.1371/journal.pone.0263512 (2022).
-
IBGE, Instituto Brasileiro de Geografia e Estatística. Pesquisa nacional de saúde: 2019 : informações sobre domicílios, acesso e utilização dos serviços de saúde: Brasil, grandes regiões e unidades da federação. Rio de Janeiro, RJ, Brasil (2020).
-
Gutierrez, S., Logan, R., Marshall, C., Kerns, J. & Diamond-Smith, N. Predictors of COVID-19 vaccination likelihood among reproductive-aged women in the United States. Public Health Rep. 137, 588–596. https://doi.org/10.1177/00333549221081123 (2022).
-
Bhattacharya, O., Siddiquea, B. N., Shetty, A., Afroz, A. & Billah, B. COVID-19 vaccine hesitancy among pregnant women: a systematic review and meta-analysis. BMJ Open 12, e061477. https://doi.org/10.1136/bmjopen-2022-061477 (2022).
-
Mendoza-Sassi, R. A. et al. Vaccination against influenza among pregnant women in southern Brazil and associated factors. Cien. Saude. Colet 24, 4655–4664. https://doi.org/10.1590/1413-812320182412.08382018 (2019).
-
Faria, A. P. V. et al. Factors associated with tetanus vaccination in pregnant women living in Minas Gerais State, Brazil: a cross-sectional study. Public Health in Practice 2, 100203, doi: https://doi.org/10.1016/j.puhip.2021.100203(2021).
-
Badell, M. L., Dude, C. M., Rasmussen, S. A. & Jamieson, D. J. Covid-19 vaccination in pregnancy. BMJ 378, e069741. https://doi.org/10.1136/bmj-2021-069741 (2022).
-
Lancet, T. COVID-19 in Brazil: “So what?”. Lancet 395, 1461. https://doi.org/10.1016/S0140-6736(20)31095-3 (2020).
-
De Brabandere, L. et al. Influence of the COVID-19 pandemic and social media on the behaviour of pregnant and lactating women towards vaccination: a scoping review. BMJ Open 13, e066367. https://doi.org/10.1136/bmjopen-2022-066367 (2023).
-
Fujita, D. M. et al. Fake news and covid-19: a concern due to the low vaccine coverage in Brazil. Saúde e Sociedade 31, doi: https://doi.org/10.1590/s0104-12902022210298 (2022)
-
IBGE, Instituto Brasileiro de Geografia e Estatística. Panorama, <https://cidades.ibge.gov.br/brasil/rj/rio-de-janeiro/panorama> (2022).
-
SMS-RJ, Secretaria Municipal de Saúde do Rio de Janeiro. SMS/SUBPAV/SVS/CAS/GTDV. Sistema de Informações sobre Nascidos Vivos -SINASC). <http://sistemas.saude.rj.gov.br/tabnet/tabcgi.exe?sinasc/nascido.def > (2021).
-
SMS-RJ, Secretaria Municipal de Saúde do Rio de Janeiro. Boletim Epidemiológico COVID-19, 2020–2022. (Centro de Operações de Emergência, Rio de Janeiro, RJ, Brazil). ).<https://coronavirus.rio/boletim-epidemiologico/> > (2022).
-
SMS-RJ, Secretaria Municipal de Saúde do Rio de Janeiro Plano Municipal de Imunização – COVID-19, 3º ed. (Centro de Operações de Emergência, Rio de Janeiro, RJ, Brazil).<https://coronavirus.rio/wp-content/uploads/2021/05/Covid_PlanoImunizacao_20210525.pdf> (2021).
-
Brasil, Ministério da Saúde. Nota Técnica nº 2/2021. (SECOVID/GAB/MS, Secretaria Extraordinária de Enfrentamento à COVID, Brasil). <https://www.gov.br/saude/pt-br/coronavirus/notas-tecnicas/2021/nt-02-2021-secovid-vacinacao-gestantes-e-puerperas-1.pdf/view(2022).
-
Yancey, W. E. (Statistical Research Division U.S. Census Bureau). U.S. Census Bureau, Washington, DC, 2005.
Acknowledgements
The authors gratefully acknowledge the Osvaldo Cruz Foundation, as the study is part of the Fiocruz VigiVac program; the Health Secretary of Rio de Janeiro City for the support, and the DATASUS for its diligent work in providing the unidentified Brazilian databases. This study was financed in part by the Coordination for the Improvement of Higher Education Personnel, Ministry of Education of Brazil (CAPES), Finance Code 001, as the author MASBB received a scholarship to conduct part of her doctoral research in the London School of Hygiene and Tropical Medicine. ESP is funded by the Welcome Trust [grant number 213589/Z/18/Z]. In addition, this study was partially supported by a donation from the “Fazer o bem faz bem” program from JBS. The study’s funders had no role in study design, data collection, analysis, interpretation, or report writing.
Author information
Authors and Affiliations
Contributions
MLB, MB-N, and ESP conceived the idea for the study. All authors contributed to the study design, with MASBB, PTVF, ESP, and TC-S drafting the statistical analysis plan. M.B.-N., VAO, and MLB organised the data linkage and secured funding. PTVF, TC-S, VAO and LFC had access to individual-level data from Brazil’s information systems and have performed data linkage. MASBB conducted the statistical analysis using the de-identified dataset, and PTVF, ESP, and TC-S accessed and verified the data analyses. All authors contributed to the interpretation of the results. MASBB, PTVF, and ESP wrote the initial draft of the manuscript. MB-N, ESP, TC-S, LFC, VAO, GMOA, RSP, DS, GLW, JMP, PSSC, MLB, MHOG, and GOP critically revised and complemented the manuscript. All authors contributed, reviewed, and accepted the final version of the manuscript for submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Borges, M.A.S.B., Florentino, P.T.V., Cerqueira-Silva, T. et al. Factors associated with COVID-19 vaccination among pregnant women in Rio De Janeiro City, Brazil.
Sci Rep 13, 18235 (2023). https://doi.org/10.1038/s41598-023-44370-6
-
Received:
-
Accepted:
-
Published:
-
DOI: https://doi.org/10.1038/s41598-023-44370-6
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.