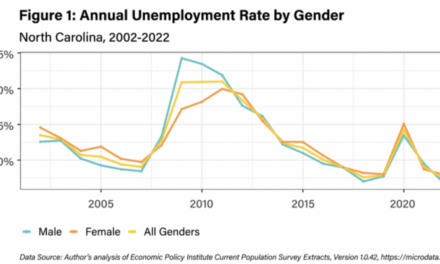
Solid tumors are famously heterogenous, giving rise to unique subpopulations that may resist one-size-fits-all treatments. For example, the aggressive tumor found in mesenchymal colorectal cancer (mCRC) does not respond to immunotherapies that are effective for other forms of colorectal cancer.
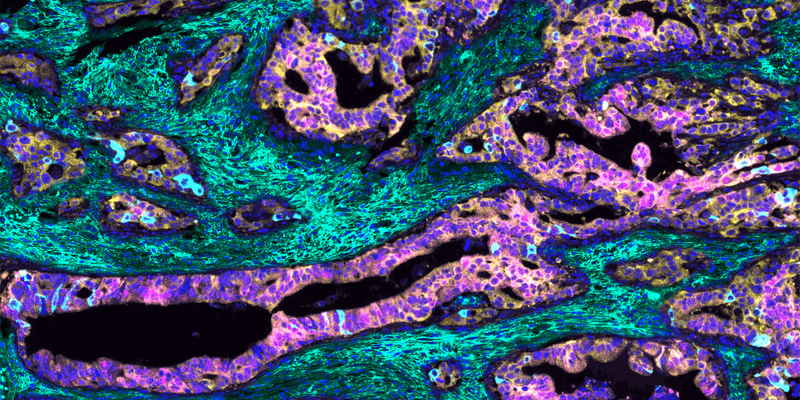
To understand what makes the mCRC tumor so unruly, cancer pathobiologists Maria Diaz-Meco and Jorge Moscat at Weill Cornell Medicine captured a multiplex immunofluorescence image (1). The researchers used this technique to paint a color-coded portrait of the tumor, enabling them to visualize, quantify, and define relationships between various cancer-associated biomolecules. Their image helped them to identify the distinct features of the mCRC tumor, unravel the mechanism that underpins its aggressive growth and immune evasion, and establish a patient selection criterion for clinical trials.
To create the multiplex immunofluorescence image, the team incubated human mCRC tissue with antibodies that bind to specific cellular proteins and extracellular molecules in the tumor environment. They then applied fluorescent dyes that stained each antibody-labeled region a different color and digitally merged them, yielding a multicolor mosaic of the tumor. “This picture is a graphical illustration showing the characteristics of these very aggressive tumors that are in the patients who have a much worse prognosis,” Diaz-Meco said.
The team observed that the aggressive mCRC cells, where unique protein markers are stained pink and light blue, do not express two yellow-stained atypical protein kinase C (aPKC) enzymes. They also found that the tumor displays a dense mesh of green-stained hyaluronan, a glucose-based polysaccharide found in the extracellular matrix. The researchers hypothesized that the cells lose the expression of the aPKC enzymes, triggering the formation of hyaluronan in the tumor environment. This hyaluronan creates a protective niche around the tumor that sustains its uncontrolled growth and shields it from the immune system. “An image can be very convincing when you are investigating a mechanism,” Diaz-Meco said. “It’s kind of trying to get the answer from the tumor itself.”
Guided by the mechanistic understanding gleaned from the image, the researchers developed a mouse model of mCRC where they used gene editing to eliminate the two aPKC enzymes, which caused hyaluronan to accumulate around the tumor. When they administered a hyaluronan-degrading hyaluronidase enzyme, they observed that the tumor shrank and became sensitized to immunotherapy.
The team plans to evaluate the activity of the hyaluronidase in a clinical trial and to acquire multiplex immunofluorescence images of patient tissue biopsy samples. More than just a pretty picture or experimental tool, these images can pinpoint individuals that possess the specific low aPKC, high hyaluronan mCRC tumor profile. “By quantitating this staining, we have this number, and we’re going to be able to say which patients qualify to respond to this therapy,” Moscat said.
The researchers are also investigating how exactly the loss of the aPKC enzymes initiates hyaluronan production, hoping to identify additional drug targets involved in hyaluronan biosynthesis. “If we can block that, we can block the tumorigenic process much earlier,” Diaz-Meco said.
References
1. Martinez-Ordoñez, A. et al. Hyaluronan driven by epithelial aPKC deficiency remodels the microenvironment and creates a vulnerability in mesenchymal colorectal cancer. Cancer Cell 41, 1-20 (2023).