Opioid use disorder (OUD) is a chronic treatable medical condition affecting birthing parents across all racial and ethnic groups, socioeconomic classes, and geographic locations. Without treatment, opioid use in pregnancy can result in serious negative health outcomes for the fetus and the birthing parent such as increased risk for a stillbirth, pregnancy complications including preterm labor, and death. Medications for OUD (MOUD) – including methadone, buprenorphine, and naltrexone – reduce adverse outcomes for both the birthing parent and child. The American College of Obstetricians and Gynecologists (ACOG) recommends the use of medications, especially opioid agonists (more details below), to manage pregnant women with OUD and advises for continued treatment through the postpartum period. Comprehensive care for pregnant and postpartum women with OUD includes standard prenatal and postpartum care, contraceptive counseling, and the co-prescribing of naloxone and overdose training.
As a major source of coverage for maternity care (covering 42% of all births), and covering an even larger share of women with OUD, and the single largest payer for behavioral health services , Medicaid is particularly well positioned to facilitate access to OUD treatment. Drawing on the 2016-2019 Medicaid claims data from the Transformed Medicaid Statistical Information Systems (T-MSIS), this brief looks at the rates of clinically documented OUD in pregnant and postpartum women as well as the percentage of diagnosed women who receive MOUD treatment. The analysis also explores disparities in clinical diagnosis and treatment based on demographics, such as race/ethnicity and age, along with geographical differences. Differences in clinically documented OUD and treatment rates across various demographics and regions offer insight to help inform ongoing policy conversations aimed at improving access to OUD treatment for pregnant and postpartum parents.
Key Takeaways:
- Analysis of Medicaid claims representing births from 2017 and 2018 in 39 states with usable data shows 2.7% of pregnant or postpartum Medicaid enrollees had clinical documentation of opioid use disorder in their medical claims. This is slightly higher than adults overall (2.0%) and lower than the adolescent and nonelderly adult (12+) Medicaid population clinically diagnosed with OUD (3.3%). There was considerable variation in the rates by states, ranging from a low of 0.4% in Nebraska to a high of 12.4% in Vermont.
- On average, 55% of pregnant and postpartum Medicaid enrollees with documented opioid use disorder received medication as part of their care. Medication treatment rates for pregnant or postpartum Medicaid enrollees with a documented OUD varied substantially with a low of 19% in Kansas to a high of 79% in Maine.
- Younger pregnant or postpartum enrollees had a clinically documented OUD rate (1.6%) that was half of those ages 26 to 34 (3.7%) and 35 years and older (3.1%). Younger enrollees received treatment at somewhat lower rates with 48% getting medication compared to over 55% among those who were aged 26 and older.
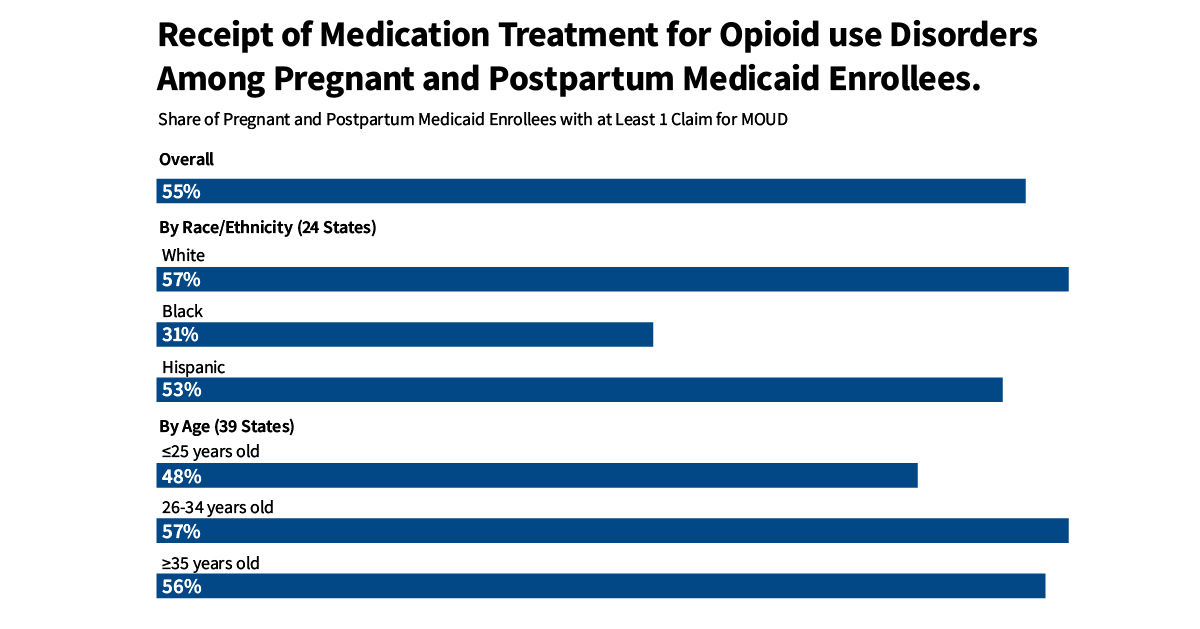
- In a subset of 24 states with available data, White pregnant or postpartum Medicaid enrollees had clinically documented OUD rates five times more than Black enrollees (5.5% vs 1.1%, respectively). Clinically documented rates were the lowest among Hispanic enrollees (0.6%). Racial and ethnic disparities persist in the receipt of MOUD. Compared to Hispanic and White enrollees, smaller shares of Black enrollees with a documented OUD received MOUD during the perinatal period (53-57% vs. 31% respectively).
- State laws that take a punitive approach toward substance use during pregnancy may contribute to lower OUD identification and lower treatment rates may be attributable in part to Medicaid utilization controls, prior authorization requirements, and burdensome administrative policies.
How are Medications for Opioid Use Disorder (MOUD) Used to Treat Opioid Used Disorder During the Perinatal Period?
Since the 1970s, ACOG has recommended that MOUD, in combination with behavioral health interventions, serves as the standard treatment for opioid addiction during the perinatal period. MOUD provides stabilization by reducing withdrawal symptoms and the negative health outcomes associated with opioid use. There are several different Food and Drug Administration-approved options for MOUD (Table 1). Methadone and buprenorphine are safe and effective in treating OUD in pregnancy and improve the adherence to standard prenatal care. Naltrexone is another treatment option for OUD, but it is rarely prescribed during pregnancy because there are few studies demonstrating its effectiveness, except in limited circumstances. While behavioral health interventions are encouraged as a supplement to MOUD, we do not discuss them in detail in the current analysis.
Without treatment, opioid use in pregnancy can result in serious negative health outcomes such as fetal distress, intrauterine growth restrictions and ultimately, neonatal abstinence syndrome (NAS) or opioid withdrawal at birth. Pregnant and postpartum parents with untreated OUD are also at increased risk for a stillbirth, pregnancy complications including preterm labor, and death. Notably, opioids played a role in 1 in 10 pregnancy-associated deaths in 2016. Although clinical guidelines recommend the use of MOUD for pregnant and postpartum parents with OUD, most go untreated. Prior studies indicate that only 50-60% of pregnant women in the United States receive any MOUD during pregnancy. In addition to this treatment gap, racial and ethnic disparities have been reported in the receipt of MOUD. Pregnant women of color are less likely to receive any medication to treat OUD.
What are the Rates and Characteristics of Pregnant and Postpartum Enrollees with a Clinically Documented OUD?
Among women with a Medicaid funded live birth in 2017 and 2018, 2.7% or 65,092 enrollees had a clinically documented OUD (Figure 1). This is slightly higher than adults overall (2.0%) and lower than the nonelderly adolescent and adult (12+) Medicaid population (3.3%). For this analysis, any diagnosis or prescription code that suggests the presence of an OUD is defined as a “clinically documented OUD.” Although, other Medicaid studies using claims data show similar rates of maternal OUD, research that adjusts for underreporting finds higher rates. Certain factors may lead to underreporting or reluctance to disclose drug use, which in turn can result in an underestimation of OUD in claims data. Stigma and concerns about legal retribution might make pregnant and postpartum women more careful about disclosing their opioid use to clinicians. Providers may be hesitant to record the diagnosis in the records at all due to concerns about whether documentation could violate the privacy rules in place that provide protection for people receiving any form of SUD treatment. For these reasons, this measure should not be used as a metric to define the overall prevalence of OUD among the pregnant and postpartum Medicaid population because not everyone is screened and diagnoses are not always recorded, but it does provide some insight into how often OUD is recognized and possibly treated in clinical settings.
Rates of clinically documented OUD in pregnant and postpartum Medicaid enrollees vary widely from state to state. For instance, Vermont has the highest share of clinically documented OUD (a finding aligning with other KFF work), with 12.4% of pregnant and postpartum Medicaid enrollees having a clinically documented OUD. In contrast, Nebraska records the lowest rate of diagnosed OUD, with fewer than 1% of pregnant and postpartum Medicaid enrollees having a clinically documented OUD (Figure 1). Rates of diagnosed OUD vary across state, not only because of prevalence, but also because of other factors such as provider screening behavior, variation in Medicaid coverage of SUD services and state laws criminalizing maternal drug use.
State laws that take a punitive approach toward substance use during pregnancy may contribute to lower OUD diagnosis and treatment. State legislation pertaining to drug use during pregnancy usually falls into punitive or supportive categories. Punitive laws categorize prenatal drug use as a form of child abuse or neglect and necessitate healthcare professionals report these instances to state child welfare agencies. These laws may deter pregnant people from seeking standard prenatal care, substance use treatment, and increase fear and concern over the potential loss of custody of their children due to the involvement of child welfare agencies. Alternatively, supportive drug use legislations attempt to prioritize pregnant and postpartum people’s access to treatment. Professional groups, such as ACOG and ASAM, oppose the use of policies and practices that criminalize drug use in pregnancy and advocate for comprehensive and evidence-based care.
Larger shares of White pregnant and postpartum Medicaid enrollees and those over the age of 25 had a documented OUD compared to Black and Hispanic pregnant and postpartum enrollees and those age 25 and younger. OUD is documented five times more in the claims of White enrollees, with 5.5% having a clinically documented OUD, compared to around 1% of Black and Hispanic enrollees. In addition, clinically documented OUD was more common among enrollees who were 26 and older (3.7% between 26-34 years old and 3.1% for those 35 years and older), compared to those aged 25 or younger (1.6%) (Figure 2). The rates at which OUD is diagnosed and documented can differ among populations due to several factors. Apart from the actual prevalence of the condition, these variations can stem from unequal access to prenatal care, inconsistencies in how providers screen for the disorder, and heightened stigma for certain groups that may amplify repercussions of admitting drug use.
What Percentage of Pregnant and Postpartum Women with a Clinically Documented OUD Received MOUD Treatment?
Overall, just over half (55%) of enrollees received MOUD as part of their care (Figure 3). Despite a little more than half of pregnant and postpartum Medicaid enrollees with a clinically documented OUD showing receipt of MOUD treatment, this is still likely an overestimate of treatment access. Research suggests that OUD prevalence is likely higher than what is reported in claims data. Yet, the number of people receiving MOUD treatment is probably fairly accurate. Therefore, the percentage of enrollees with opioid use disorder – whether documented in claims data or not – who receive treatment is likely lower than 55%.
State treatment rates of pregnant and postpartum Medicaid enrollees with a documented OUD also vary substantially. States where more than 60% of enrollees with a clinically documented OUD received MOUD included Northeastern states (ME, VT, NH, MA, CT, DE) and WA, NM, WI, and OH. On the other hand, in KS, less than 1 in 5 received MOUD. Please refer to Methods and Appendix 2 for more information.
Rates of MOUD treatment vary across states because of factors such as Medicaid utilization controls and variation in engagement in public health initiatives like the development of perinatal collaboratives focusing on OUD throughout the perinatal period or the receipt of SAMHSA grants. Utilization control measures, like prior authorization, shape the access and availability of MOUD and can be a barrier to care, particularly if prior authorization denial rates are high. These measures include prior authorization, quantity limits, step therapy, and psychosocial treatment requirements. In addition to prior authorization requirements, some states also have additional administrative requirements, which may include random drug screenings or pill counts, mandatory counseling requirements, and maximum daily doses.
Additionally, MOUD utilization and adherence are affected by treatment concerns and scarcity of women- centered programs. Prior research finds that pregnant and postpartum individuals express concerns about lack of autonomy in their decision to initiate MOUD and have felt pressured from clinicians, ultimately impacting their commitment to treatment. Other pregnant and postpartum individuals voice concerns about their infants developing NAS and the increased scrutiny they would receive from healthcare staff. Lastly, postpartum parents have highlighted that treatment environments for MOUD are not accommodating to their unique needs. Because methadone and buprenorphine are subject to federal and state restrictions limiting their accessibility, postpartum parents have highlighted the difficulties in maintaining adherence with competing childcare responsibilities. Other challenges affecting adherence in the postpartum period include cost, transportation, lack of continued healthcare coverage after delivery, and shortage of treatment centers and clinicians providing MOUD. Research has shown that women-centered programs – programs that offer services tailored to women’s unique needs – have higher retention rates and reductions in substance use, and fewer reported barriers to care.
Black women with a diagnosed OUD were less likely to receive MOUD during prenatal or postpartum periods (31%) compared to Hispanic and White women (53-57% respectively). However, treatment rates were similar between White and Hispanic pregnant and postpartum enrollees. Research suggests that structural racism may be associated with lower standards of care, fewer treatment options, and higher rates of prosecution of women of color, especially Black women. Black women who use drugs are more likely to be reported to child welfare agencies and drug tested than other women. These factors may make women of color less likely to disclose opioid use and contribute to lower treatment rates. Additionally, the absence of a diverse healthcare workforce may result in reluctance to seek or continue treatment. Prior studies have also drawn attention to the limited diversity of workforce in outpatient substance use treatment settings. Furthermore, the existing knowledge base may not be reflective of the experiences of women of color, with previous research noting that White women with an OUD made up most of their sample. Regarding age, pregnant and postpartum women 26 years and older (56-57%) with diagnosed OUD were more likely to receive MOUD treatment compared to those 25 years old and younger (48%) (Figure 4).
What Changes have been made at the Federal and State Level to Support Pregnant and Postpartum Individuals Diagnosed with Opioid Use Disorder?
In recent years, state and federal governments have undertaken additional actions to address gaps in treatment, prevention, and recovery of substance use disorder services. In 2018, the Substance Use Disorder Prevention that Promotes Opioid Recovery and Treatment for Patients and Communities (SUPPORT Act), a bipartisan legislation, was passed that included Medicaid provisions to expand research and services for pregnant women with OUD. At the same time, CMS launched the Maternal Opioid Misuse (MOM) Model Initiative to address fragmentation in the care of pregnant and postpartum Medicaid enrollees with OUD. There are currently 8 states participating in the program and interventions focus on service integration, coordination, and expansion to ultimately improve the cost, quality, and access to OUD services. In 2021, states were given the option to extend Medicaid postpartum coverage to 12 months in the American Rescue Plan Act and most states have implemented or plan to implement a 12-month extension. This 12-month postpartum extension gives postpartum Medicaid enrollees more time to receive care, ensuring longer access to OUD treatment services. More recently, the Consolidated Appropriations Act of 2023 led to the elimination of additional registration requirements for the prescribing of buprenorphine (X waiver) to facilitate access to buprenorphine providers; funding support to expand maternal mental health screening programs; maintenance of maternal mental health hotline; and establishment of a maternal mental health task force.
Looking Ahead
Although federal policies have aimed to improve access to OUD, treatment gaps persist. Most recently, the resumption of Medicaid renewals following a three-year pandemic halt – termed ‘Medicaid unwinding’ – has led to many individuals being dropped from Medicaid, primarily due to procedural rather than eligibility reasons. While some people losing Medicaid may already have another source of coverage or be able to transition to another form of insurance like the Affordable Care Act marketplace, other pregnant and postpartum women who qualify for Medicaid through pathways other than a current or recent pregnancy may experience coverage loss. Such a loss may disrupt treatment for OUD, increasing overdose risks, especially in the midst of the ongoing fentanyl crisis. If Medicaid unwinding coverage losses disproportionately affect people of color, it could intensify existing racial and ethnic disparities in access to MOUD.
The authors would like to acknowledge Mishka Terplan, MD, MPH, a Medical Director at Friends Research Institute and adjunct faculty at University of California, San Francisco for his review of earlier drafts of this brief and the Urban Institute for their provision of the Behavioral Health Services Algorithm (BHSA).