Key Takeaways
- Amyloid Hypothesis and Novel Monoclonal Antibodies: Amyloid beta protein accumulation in the brain is a key factor in Alzheimer disease (AD) pathogenesis, and novel monoclonal antibodies such as aducanumab, lecanemab, and donanemab aim to slow this process by reducing amyloid beta plaques.
- Treatment Paradigm Shift and Monitoring Challenges: Aducanumab, lecanemab, and donanemab represent a shift in AD treatment, demonstrating a potential to slow disease progression, but their use necessitates careful monitoring for amyloid-related imaging abnormalities and other potential adverse effects.
- Considerations for Pharmacists in Patient Care: Pharmacists play a crucial role in ensuring appropriate dosing, administration, monitoring, and counseling for patients receiving these novel monoclonal antibody therapies for AD, considering individualized risk factors and benefits to optimize patient outcomes.
Alzheimer disease (AD) is a neurodegenerative disorder that affects millions of Americans.1 Early signs of AD include memory loss, mood changes, taking longer to complete daily tasks, experiencing challenges when attempting to problem solve, getting lost, and misplacing items.2
Image credit: Dr_Microbe | stock.adobe.com
AD is often diagnosed at this stage; however, it is hypothesized that changes in the brain may begin up to 10 years before symptoms start to appear.1 One of these abnormal findings in the brain is the buildup of amyloid beta proteins that form plaques and cause toxic damage to neurons, which is known as the amyloid hypothesis.1
Even though it is one of the most widely accepted models to explain the pathogenesis of AD, drug development in this area has yet to be successful.3 Current drug therapy options for AD patients are only beneficial for managing the symptoms of AD and are not able to slow disease progression.
These options have remained the same for nearly 2 decades, warranting a need for new medications. Aducanumab, lecanemab, and donanemab are novel monoclonal antibodies designed to slow this process and hopefully have a positive impact in the field of AD management.
They are the first treatment options for AD that have demonstrated slowing of AD-related decline by supporting the amyloid hypothesis. They work by binding to soluble amyloid beta peptides in the periphery and sequestering them into a complex that can be removed from the circulation.
These agents reduce the formation of amyloid beta plaques and prevent them from depositing into the brain.4 All 3 monoclonal antibodies require monitoring for amyloid-related imaging abnormalities (ARIA), which is divided into 2 subtypes, ARIA-E for edema and ARIA-H for hemosiderin or hemorrhage (although they can coexist).5
Although the exact mechanism of these abnormalities is unknown, risk factors include high doses of anti-amyloid monoclonal antibodies and presence of apolipoprotein E ε4 (ApoE ε4) alleles. ARIA is usually asymptomatic but can lead to new signs and symptoms such as headache, altered mental status, dizziness, visual disturbances, nausea, and seizures.5
Aducanumab (Aduhelm)
Aducanumab was FDA approved in June 2021. It is indicated for the treatment of AD in patients with mild cognitive impairment or dementia with confirmed presence of amyloid beta pathology prior to treatment initiation.5
Dosing and Administration
Dosage Forms: Intravenous solution, aducanumab-avwa 300 mg/3 mL,170 mg/1.7 mL.
Dosing (based on actual body weight): Administer infusions at least 21 days apart.
- Infusions 1 and 2: 1 mg/kg once every 4 weeks
- Infusions 3 and 4: 3 mg/kg once every 4 weeks
- Infusions 5 and 6: 6 mg/kg once every 4 weeks
- Maintenance dose: 10 mg/kg once every 4 weeks (starting with infusion 7)
Dosing Adjustments
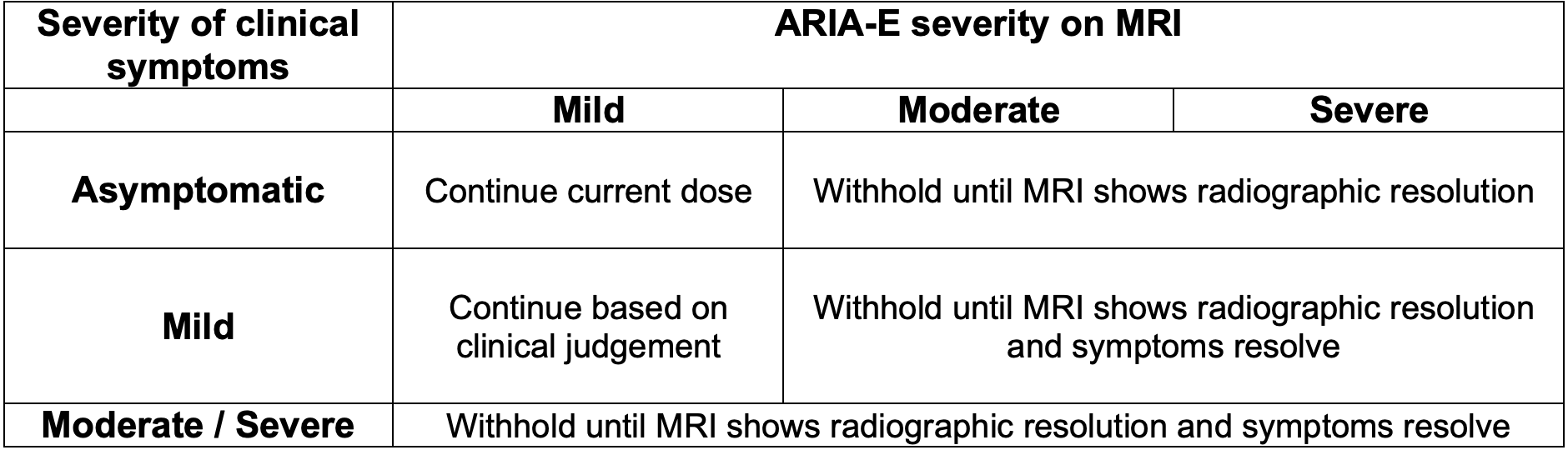
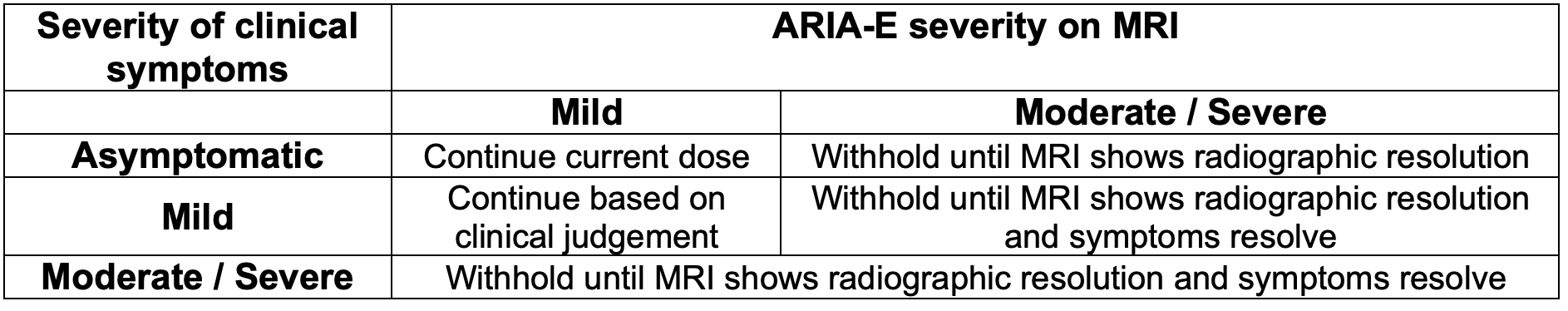
There are currently no recommended adjustments for renal or hepatic dysfunction. The recommendations for modifying aducanumab therapy are related to ARIA-E and ARIA-H.
If withholding therapy due to these abnormalities, consider a follow up MRI in 2-4 months. Clinicians may resume therapy at the same dose prior to withholding, as guided by clinical judgement.
For guidance regarding management of ARIA-E and ARIA-H based on MRI findings, refer to the tables below.

Administration
Dilution required prior to administration. Allow refrigerated infusion bags to warm to room temperature prior to administering. Administer by IV infusion over ~60 minutes through an IV line containing a sterile, low-protein binding, 0.2 or 0.22 micron in-line filter. Discard unused portion.
Warnings and Precautions
- Polysorbate 80: Some dosage forms may contain polysorbate 80 (also known as Tweens). Hypersensitivity reactions, usually a delayed reaction, have been reported following exposure to pharmaceutical products containing polysorbate 80 in certain individuals.
- Safety and effectiveness of initiating treatment at stages other than mild cognitive impairment have not been studied. Confirm the presence of amyloid beta pathology prior to treatment initiation.
Monitoring Parameters
- Prior to initiation: PET or lumbar puncture to confirm presence of amyloid beta pathology.
- Brain MRI: Prior to initiation (within the past year), prior to infusion 5, infusion 7, infusion 9, infusion 12, and as indicated if ARIA symptoms present or asymptomatic ARIA observed.
- Clinical symptoms and MRI changes
- Symptoms suggestive of ARIA such as headache, altered mental status, dizziness, visual disturbance, seizure, nausea
Counseling Points
Adverse effects (AEs): ARIA, increased risk of falls, headache, diarrhea, altered mental status, confusion, delirium
Drug-drug interactions: Aducanumab has the potential to diminish the effectiveness of Fc receptor binding agents, such as efgartigimod alfa and rozanolixizumab.
Safety and Efficacy
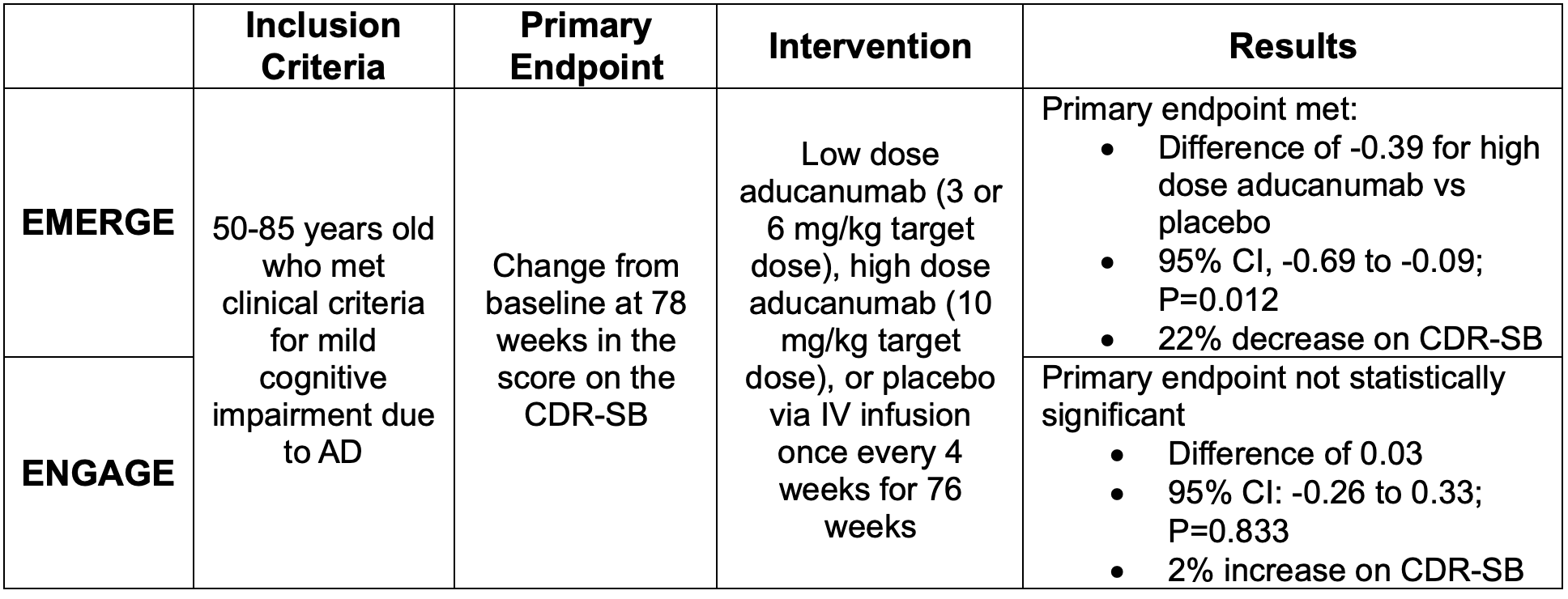
Safety and efficacy of aducanumab in early AD was evaluated in two randomized, double-blind, placebo-controlled trials titled EMERGE and ENGAGE.6 The primary outcome was change from baseline on the Clinical Dementia Rating Sum of Boxes (CDR-SB) at 78 weeks.
This scale ranges from 0 to 18 and assesses both function and cognition. Higher scores indicate greater impairment.7 Both of these studies were terminated early due to meeting prespecified futility criteria for inefficacy during the interim analysis. The trial results and observed incidence of ARIA are outlined below.

Lecanemab (Leqembi)
Lecanemab was approved by the FDA in January 2023. It is indicated for treatment of AD in patients with mild cognitive impairment.5
Dosing and Administration
Dosage Forms: Intravenous solution, lecanemab-irmb 200 mg/2 mL, 500mg/5mL
Dosing (based on actual body weight): 10 mg/kg once every 2 weeks
Dosing Adjustments: There are currently no recommended adjustments for renal or hepatic dysfunction. The recommendations for modifying lecanemab therapy are related to ARIA-E and ARIA-H.
If withholding therapy due to these abnormalities, consider a follow up MRI in 2-4 months. Clinicians may resume therapy at the same dose prior to withholding, as guided by clinical judgement.

Administration
Dilution required prior to administration. Administer by IV infusion over 1 hour through an IV line containing a terminal low-protein binding 0.2 micron in-line filter. Flush IV line following infusion.
Warnings and Precautions
- ARIA: Patients may develop ARIA-H with concomitant ARIA-E. The majority of ARIA-E events occur within the first 7 doses; however, ARIA may occur more than once and at any time during treatment. Use caution in patients with preexisting risk factors for intracerebral hemorrhage.
- Infusion reaction: Lecanemab may cause infusion reactions; symptoms include fever, flu-like symptoms, nausea, vomiting. The highest incidence of infusion reactions occurs with the first infusion.
Monitoring Parameters
- Prior to initiation:
- PET or lumbar puncture to confirm presence of amyloid beta pathology
- Apolipoprotein E ε4 (ApoE ε4) status testing
- Brain MRI
- Brain MRI: Prior to infusion 5, infusion 7, infusion 14, and as indicated if ARIA symptoms are present.
- Clinical symptoms and MRI changes.
- Symptoms suggestive of ARIA such as headache, altered mental status, dizziness, visual disturbance, seizure, nausea.
Counseling Points
AEs: ARIA, infusion-related reaction, atrial fibrillation, headache, diarrhea
Drug-drug interactions:
- Agents with antiplatelet properties (e.g., P2Y12 inhibitors, NSAIDs, SSRIs), anticoagulants, thrombolytic agents.
- Lecanemab may increase the toxic effect of these agents, specifically increasing risk of hemorrhage.
- Lecanemab has the potential to dimmish the effectiveness of Fc receptor binding agents (such as efgartigimod alfa and rozanolixizumab).
Safety and Efficacy
Lecanemab was studied during the CLARITY-AD trial, an 18-month, multicenter, double-blind, phase 3 trial.8 The mean CDR-SB score at baseline was approximately 3.2 in both the lecanemab and placebo groups, findings consistent with early AD (score of 0.5 to 6). Results for the primary endpoint and incidence of ARIA are outlined below.

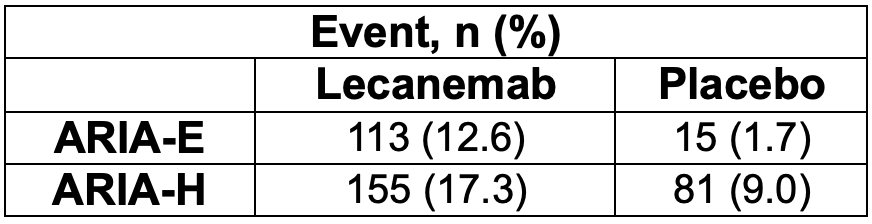
Results for the secondary endpoints trended in the same direction as the primary endpoint. After 18 months of treatment in the amyloid sub study, the mean amyloid level of 22.99 centiloids in the lecanemab group was below the threshold for amyloid positivity of approximately 30 centiloids. Additionally, in the CSF substudy, markers of amyloid, tau, neurodegeneration, and neuroinflammation were reduced to a greater extent with lecanemab compared to placebo.
Donanemab
Donanemab is not currently FDA approved. A phase 2 trial, TRAILBLAZER-ALZ was published in 2021 and a follow-on study involving the same patients, TRAILBLAZER-EXT, is currently in progress to further assess safety and efficacy via video visits. TRAILBLAZER-ALZ 2 was a phase 3 trial that published results in July 2023.9,10
Dosing and Administration
Similar to aducanumab and lecanemab, donanemab is an intravenous infusion. In the phase 2 trial, donanemab was dosed at 700 mg monthly for the first 3 months, then 1400 mg monthly for up to 18 months. Dosing adjustments have not yet been established.
Warnings and Precautions
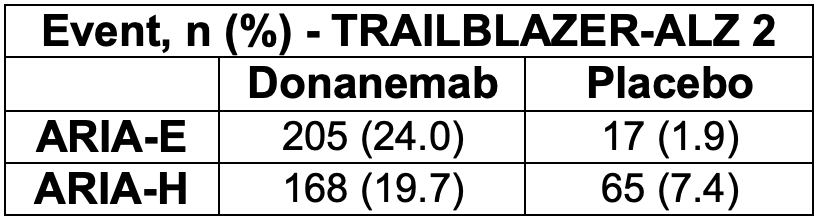
There are not any published warnings at this time, but ARIA-E occurred in approximately 1 in 4 participants in the donanemab group during TRAILBALZER-ALZ. There was a higher incidence of ARIA-E among apolipoprotein ε4 carriers, a finding similar to observations in the lecanemab trial.
Monitoring Parameters
- Prior to initiation: PET or lumbar puncture to confirm presence of amyloid beta pathology
- Prior to initiation: Apolipoprotein E ε4 (ApoE ε4) status testing
- Brain MRI (as monitored in the trials): At 4, 12, 24, 52, and 76 weeks, and as indicated if ARIA symptoms are present.
- Clinical symptoms and MRI changes.
- Symptoms suggestive of ARIA such as headache, altered mental status, dizziness, visual disturbance, seizure, nausea
Counseling Points
AEs (as seen in the trials): ARIA, nausea, urinary tract infection, diarrhea, cerebral microhemorrhage, infusion-related reaction, vomiting, anxiety
Safety and Efficacy
In trials evaluating donanemab, the primary outcome was the change from baseline in the score on the Integrated Alzheimer’s Disease Rating Scale (iADRS) at 76 weeks. The scale ranges from 0 to 144 with lower scores indicating greater cognitive and functional impairment.9,10
Trial results and incidence of ARIA are outlined below. In TRAILBLAZER-ALZ 2, donanemab treatment resulted in clinically meaningful benefit (considered to be >20% slowing of clinical progression) on the iADRS and CDR-SB scales. Furthermore, an estimated 47% of participants receiving donanemab had no change in the CDR-SB at 1 year (no disease progression), compared with 29% of participants receiving placebo.

Conclusion
Aducanumab, lecanemab, and donanemab are novel drug therapies for AD that followed a period in which there was a lack of new developments in the field. These medications have shown promise in targeting the formation of amyloid beta plaques, but do not come without risks.
It will be imperative for clinicians and patients to weigh the risks and benefits of these monoclonal antibodies before initiating therapy. Research to further confirm the safety and efficacy of these agents is ongoing.
About the Authors
Angie Giglione, PharmD Candidate 2024, University of Missouri-Kansas City – Kansas City, MO and Mary Beth Dameron, PharmD, BCACP, University Health – Kansas City, MO.
References
- National Institute on Aging. “Alzheimer’s Disease Fact Sheet.” National Institute on Aging, 5 Apr. 2023, www.nia.nih.gov/health/alzheimers-disease-fact-sheet.
- National Institute on Aging. “What Are the Signs of Alzheimer’s Disease?” National Institute on Aging, National Institute on Aging, 18 Oct. 2022, www.nia.nih.gov/health/what-are-signs-alzheimers-disease.
- Paroni, Giulia, et al. “Understanding the Amyloid Hypothesis in Alzheimer’s Disease.” Journal of Alzheimer’s Disease, vol. 68, no. 2, 29 Mar. 2019, pp. 493–510, content.iospress.com/articles/journal-of-alzheimers-disease/jad180802, https://doi.org/10.3233/jad-180802.
- Shi, Mingchao, et al. “Impact of Anti-Amyloid-β Monoclonal Antibodies on the Pathology and Clinical Profile of Alzheimer’s Disease: A Focus on Aducanumab and Lecanemab.” Frontiers in Aging Neuroscience, vol. 14, no. 1, 12 Apr. 2022, https://doi.org/10.3389/fnagi.2022.870517.
- Lexicomp. (2019). Lexi.com. https://online.lexi.com/lco/action/home
- Budd Haeberlein S, Aisen PS, Barkhof F, et al. Two Randomized Phase 3 Studies of Aducanumab in Early Alzheimer’s Disease. J Prev Alzheimers Dis. 2022;9(2):197-210. doi:10.14283/jpad.2022.30
- “CDR® Scoring Table | Knight Alzheimer Disease Research Center | Washington University in St. Louis.” Knightadrc.wustl.edu, knightadrc.wustl.edu/professionals-clinicians/cdr-dementia-staging-instrument/cdr-scoring-table/#:~:text=The%20Global%20CDR%20Score%20is%20useful%20for%20characterizing. Accessed 25 Sept. 2023.
- van Dyck, C. H., Swanson, C. J., Aisen, P. et al. (2022). Lecanemab in Early Alzheimer’s Disease. New England Journal of Medicine, 388(1). https://doi.org/10.1056/nejmoa2212948
- Mintun, Mark A., et al. “Donanemab in Early Alzheimer’s Disease.” New England Journal of Medicine, vol. 384, no. 18, 13 Mar. 2021, https://doi.org/10.1056/nejmoa2100708.
- Sims, John R., et al. “Donanemab in Early Symptomatic Alzheimer Disease: The TRAILBLAZER-ALZ 2 Randomized Clinical Trial.” JAMA, 17 July 2023, jamanetwork.com/journals/jama/fullarticle/2807533?guestAccessKey=855d51a0-c676-46f8-8bb3-1ecaa471d5eb&utm_source=For_The_Media&utm_medium=referral&utm_campaign=ftm_links&utm_content=tfl&utm_term=071723, https://doi.org/10.1001/jama.2023.13239.